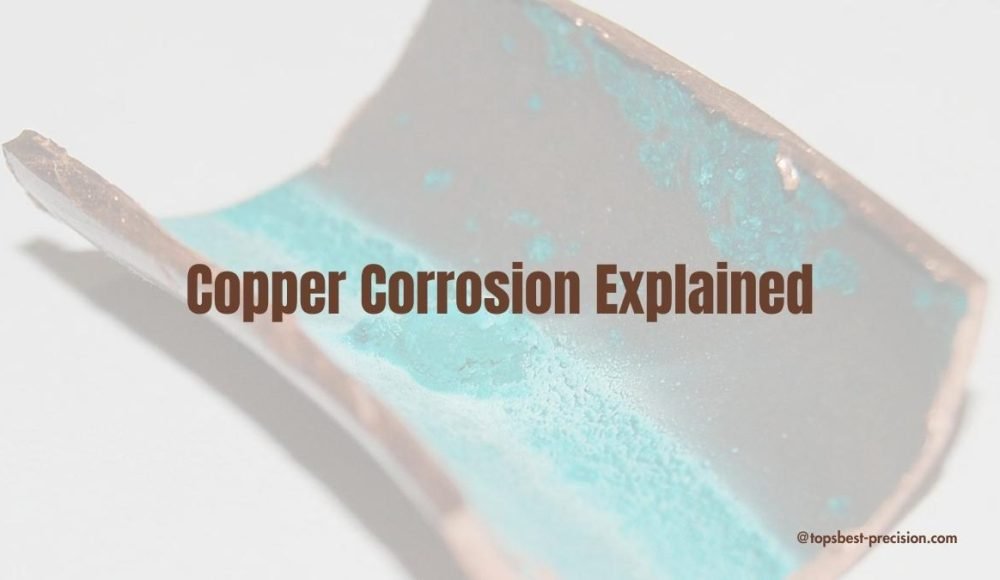
Корозията на медта означава влошаване на медта поради влага, и излагане на въздух. Типично, медта корозира, и се превръща в тъмно черно( зелен филм). Продължете да четете, за да научите повече за корозията на медта.
Има медна ръжда?
Медта не ръждясва като желязото. Терминът ръжда подчертава само продуктите от корозия на желязо. Мед, от друга страна, корозира и образува зелен слой(Патина). Общо взето, патината е меден карбонат CuCO₃. Образува се, когато Cu реагира с въздух, и вода след дълго излагане. Слоят от патина може да изглежда непривлекателен, но служи за защитна цел. Предотвратява по-нататъшна корозия на основата(лежащ в основата) метал. В резултат на това, медта може да служи дълго време с минимална поддръжка.
Как възниква образуването на медна патина?
Медта реагира с атмосферния кислород, за да образува меден оксид. Медната ръжда е червеникаво-кафяв слой от Cu2O. Този слой се развива чрез процеса на електрохимично взаимодействие с въздуха. Докато желязото се превръща в ръжда, медта не корозира, или както просто казано, не ръждясва, окислява се. Кислородът действа върху медта, за да предотврати образуването на съединение, известно като меден оксид. Този оксид има свойство, различно от това на железния оксид Fe2O3. Меден оксид, обратно, не корозира толкова бързо, колкото ръждата. Той действа като бариера, която постепенно става по-дебела, докато се натрупва. Накрая, този слой се променя в меден карбонат в този процес. Най-горният слой е известен като патина.
Фактори, допринасящи за корозията на медта
Корозията на медта може да бъде повлияна от няколко фактора. Много е наложително да знаете тези фактори, за да ги избегнете и минимизирате.
Излагане на околната среда
Корозията на медта се ускорява в области със соленост, топлина, и спорни химикали като киселини. Тези условия причиняват химическо действие с медта, което води до отслабване на повърхността и излагане на повече вреда от преди.
Ефекти на електрически ток
Въпреки че както постоянният, така и променливият електрически ток могат да повишат скоростта на корозия на медните тръби. Тези тръби често са заровени под земята. Наличието на електрически потенциални разлики влошава локализираните корозионни явления.
Галванична корозия
Галваничната корозия е мястото, където медта реагира с различни материали. например, стомана. Такова взаимодействие дава образуването на електрохимична клетка. Това осигурява по-бърза скорост на корозия на медта, тъй като проводимостта също не е еднаква.
Агресивни почвени условия
Хлориди, сулфати, а амонячните съединения обикновено присъстват в почвите. Тези естествени съставки могат да представляват голяма опасност за корозията на медта. Мобилизиране на тези агресивни почвени условия, съчетано със задържане на влага, неизменно води до сериозни загуби от корозия за известно време.
Киселинен контакт
Наличието на органични и неорганични киселини представлява голяма заплаха за качеството на медта. Тези киселини преминават през оксидния слой и продължават да разрушават материала с по-бърза скорост. Q
Умора от корозия
Умората се причинява от колебанията на напрежението, които възникват в медните материали. Бързият поток от течности и колебанията в температурите насърчават локализирана ерозия, която води до ранно разрушаване на материала.
Излагане на кислород
Степента на окисляване на медните повърхности вероятно ще се увеличи с повишаване на нивата на кислород в атмосферата. Това ускорява образуването на корозивни съединения чрез едновременно влошаване на вече лошата механична цялост на метала.
Видове корозия на медта
Медта може забележимо да коротира по определени начини. И двата вида се влияят от околната среда и химичните условия.
Равномерна корозия
Равномерната корозия се появява постепенно по повърхността и засяга еднакво цялата повърхност в даден момент. Този тип най-често води до постепенно намаляване на дебелината на метала. Това е особено често, когато медта е изложена на влага. Киселинните условия също увеличават скоростта на този равномерен процес на разграждане с влакната.
Точкова корозия
Точковата корозия причинява образуването на вдлъбнатини върху медната повърхност. Главно, провокира се от йони като хлориди с агресивен ефект. Тези ями могат да станат много дълбоки до точка, в която може да се случи структурната здравина на ямите. Точковата корозия обикновено се влошава от наличието на застояла вода.
Корозия на цепнатини
Корозията на цепнатините се случва в тесни пространства и тесни пролуки. Състоянието позволява образуването на статични решения, и изрично ограничават достъпа на кислород. При такива обстоятелства, в системата се натрупват корозивни агенти. В резултат на това, протича концентрирана атака, водеща до локализирана корозия. Най-често се случва около крепежни елементи, уплътнения, и тръбни съединения.
Галванична корозия
Галваничната корозия е фаза, при която медта се сблъсква с други видове метали. Контактът развива галваничен елемент в присъствието на електролит(проводим разтвор). Този от двата метала с по-висока реактивност корозира по-бързо. въпреки това, трябва да имате предвид, че медта може да корозира локално, което ще намали полезната дължина на материала.
Обезцинкване
Обезцинкването се случва само при месинг. Месингът е смес (сплав) от мед и цинк. По време на децинкификация, една от двойките или компонентите, винаги се вижда, че цинковите елементи се разпадат. Това води до пореста структура на медта, която е сравнително по-слаба от първоначалната структура. Обезцинкването е особено нежелателно в приложения, където се изисква висока степен на механични характеристики.
Междукристална корозия
Междукристална корозия се извършва по границите на зърната в медта. Типът обикновено произлиза от неадекватни процеси на топлинна обработка по време на производството. То може също да бъде насърчено от излагане на определени корозивни условия, както е обяснено по-горе. Отслабването на материала може да доведе до катастрофални повреди.
Корозионно напукване под напрежение
Корозионното напукване под напрежение е продукт на напрежение и корозивен агент на повърхността на материала. Той образува някои малки отвори в медния материал, обикновено наричани разкъсвания. Обикновено, амонякът допринася за този проблем. освен това, високите температури също могат да доведат до проблеми с разрушаването.
Различните форми на корозия на медта се характеризират с различни трудности и процеси. Това са аспекти, които трябва да вземем предвид – и да разберем – за да знаем как да процедираме по въпроса за превенцията. Корозията се влияе основно от физич, и химични свойства на медните сплави, условия на околната среда, и упражняваните напрежения.
Примери за устойчиви на корозия сплави
Алуминиев месинг: Тези сплави са много имунизирани срещу ударна корозия, особено при приложения с висока скорост на солена вода.
Алуминиев бронз: Използва се широко заради способността си да устои на химически атаки, особено от сулфитни разтвори.
Медно-силициеви сплави: Тези сплави предлагат висока устойчивост на корозионно напукване в сравнение с конвенционалния месинг.
Никелово сребро: Те са склонни да осигуряват отлична устойчивост срещу корозия както от прясна, така и от морска вода.
Заключение
В обобщение, наложително е да запазите медта и да разберете как тя корозира. Корозията се появява в много форми, вариращи от образуване на пукнатини до слой патина. И двете униформени, костилка, и галваничната корозия представляват специфични видове корозия и изискват специфични мерки за тяхното смекчаване. По това, можем да идентифицираме какво води до корозия и след това да разберем как да я избегнем. Като цяло, информацията относно механизма на корозия на медта не само помага за запазването на великолепната архитектура и реликви. Също, спомага за повишаване на ефективността на използването на мед в различни области.
Често задавани въпроси
Q1. Какво е корозия на медта, и неговите причини?
Корозията на медта се отнася до загуба на свойствата на материала. Медта се дължи на химическото действие на околната среда, при което тя разпределя компонентите си под въздействието на влага и въздух. Процесът образува зеленикава патина върху металната повърхност.
Q2. Как се образува патината върху меден материал?
Медната патина се образува, когато медта се окислява от кислород, въглероден диоксид, и влага. Допълнителният слой патина помага да се спре по-нататъшната корозия, ключова част от издръжливостта на медта на открито.
Q3. Може ли да се предотврати корозията на медта?
да, защитните мерки включват нанасяне на антикорозионни бои, използването на забавители на корозията, и осигуряване на подходящ дренаж за минимизиране на условията. Други превантивни действия включват измиване на мед, и полиране за подобряване на външния вид и дълголетието на медната повърхност.
Q4. Как корозията на медта влияе върху строителния сектор?
Когато медта корозира, това може да отслаби конструкции в области като водопровод и покриви. Ето защо е важно да се разбира и контролира корозията, за да се удължи живота и безопасността на структурите на частите.