金属は一般に特性と関連しています, つまり. 強さ, 耐久性, そして剛性. しかし, すべてではありません 金属 一生懸命に考えることができます. 金属の中には信じられないほど柔らかいものもあります, つまり、アプリケーションが非常に特殊であることを意味します. このうち, 最も興味深いのは、硬度が低いという点で特別です。, 展性, と延性. それで, 記事上で, 私たちは事実を明らかにし、地球上で最も柔らかい金属が何かを説明します. このほかにも, 生活のさまざまな分野におけるその数多くの応用について説明します.
地球上で最も柔らかい金属は何ですか?
セシウムは地球上で最も柔らかい金属であり、アルカリ金属グループの一部です. それで, 他の金属より反応性が高い. このほかにも, ナイフで削り取ることができます. 融点について言えば, 83.3°Fです (28.5℃). それで, さまざまな形状に簡単に成形できます.
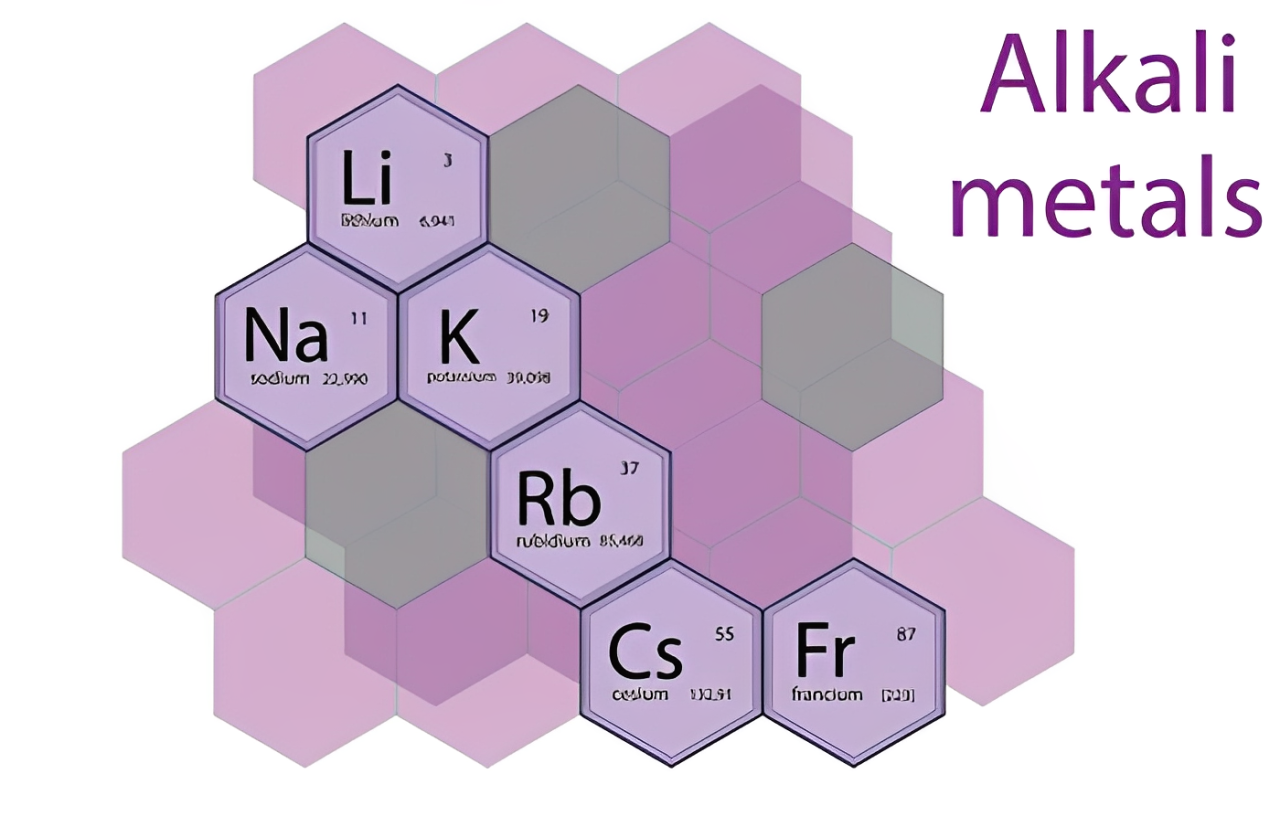
セシウムはリストの中で最も柔らかい金属です, リチウムと一緒に, ナトリウム, そしてカリウム. アルカリ金属は反応性が高く、比較的軽量です。, これらすべての要素には多くの共通の特性があります. しかし, セシウムは最も柔らかい元素と言えます, 周期表以外のいくつかの方法を示す.
セシウムの物理的および化学的特性
それで, 地球上で最も柔らかい金属であるセシウムの特徴をいくつか紹介します。:
1. 物理的特性
その物理的特性について話しましょう:
- 外観: セシウム, 標準状態で, 金色の色合いを持つほとんどの金属と同じように光沢のある銀色です.
- 密度: まだ, その密度, 1.93 g/cm3, 比較的低密度の金属にセシウムを入れる.
- 融点: わずかに暖かい条件下では簡単に溶けます; 28.5℃以下の温度では固体になります (83.3°F) このくらいともう少し温度を上げると溶けます.
- 柔らかさ: セシウムは展性がある, 一般的なリードのように, 簡単に手で形を整えることができます, 反応性が非常に高いため扱うことはできませんが、.
2. 化学的特性
その化学的性質は次のとおりです:
- 反応性: この元素は特に水に対して非常に反応しやすいです, それと結合して爆発的な反応を起こす.
- アルカリ金属グループ: グループ内にあります 1 周期表の周期表に属しており、イオン化エネルギーが低く、電気陽性率が高いなどの特徴を持っています。.
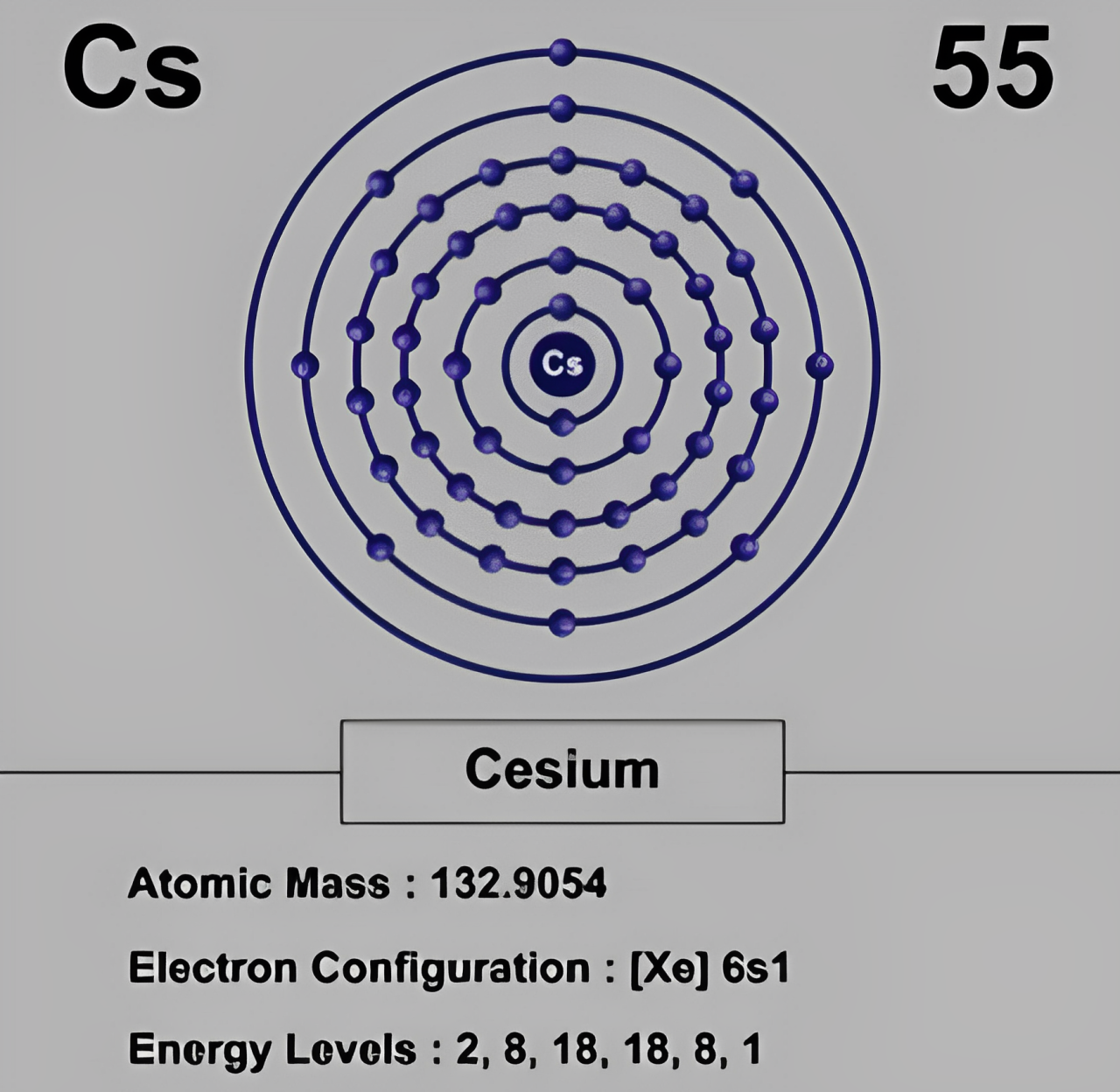
- 電子配置: セシウムには価電子が1つしかありません. それで, セシウムはイオン結合を形成する傾向が強い.
これらの機能を次の表にまとめます。:
| 特徴 | 値/説明 |
| 物理的特性 | |
| 外観 | シルバーゴールドの金属光沢 |
| 密度 | 1.93 g/cm3 |
| 融点 | 28.5℃ (83.3°F) |
| 柔らかさ | 非常に柔らかい; 手で成形できる (反応性があるため慎重な取り扱いが必要です) |
| 化学的特性 | |
| 反応性 | 反応性が高い; 水との爆発的反応 |
| アルカリ金属グループ | グループに属しています 1 周期表の |
| 電子配置 | [車] 6s¹ (1つの価電子, 反応性が高く、イオン結合を形成する) |
金属の柔らかさ – セシウムが最も柔らかい金属である理由
セシウムは原子の性質により、すべての元素の中で最も柔らかいものとなっています。. 原子の実効原子番号は、原子サイズが大きいため、セシウムの最外殻電子は原子核から非常に遠い位置にありました。. それで, これにより、材料を構成する原子間の金属結合が減少します。. そうするととても柔らかくなりますよ.
他の軟金属との比較
それで, セシウムと他の軟アルカリ元素を比較してみよう:
1. リチウム (李)
- 柔らかさ: ナイフでスライスできますが、セシウムよりも硬いです.
- 反応性: 大部分の金属よりも反応性が高い, まだセシウムほど反応性はない.
- 用途: 電池と合金.
2. ナトリウム (すでに)
- 柔らかさ: リチウムよりも展性が高いが、セシウムよりは展性が低い.
- 反応性: 水と激しく反応する.
- 用途: ナトリウムランプ, 化学合成 ナトリウムランプ 化学合成
3. カリウム (K)
- 柔らかさ: ヤスリで表面をこすってもよい, しかし、鉛よりも硬く、セシウムよりも柔らかくありません.
- 反応性: 熱を伴う水との激しい反応と水素ガスの発生.
- 用途: 耕地と化学的改良.
それで, セシウムはこれらの金属とは異なり、非常に柔らかく、独特の黄金色をしています。.
これは、地球上で最も柔らかいさまざまな金属の比較をまとめた表です。:
| 金属 | 柔らかさ | 反応性 | 用途 |
| リチウム (李) | セシウムより硬い, ナイフで切れる | 非常に反応性が高いがセシウムより低い | 電池, 合金 |
| ナトリウム (すでに) | リチウムより柔らかい, セシウムより硬い | 水と激しく反応する | ナトリウムランプ, 化学合成 |
| カリウム (K) | ナトリウムよりも柔らかい, セシウムより硬い | 反応性が高い, 水から熱と水素を生成する | 肥料, 化学反応 |
| セシウム (Cs) | 最も柔らかい金属, 容易に成形可能 | 非常に反応性が高い, 特に水の場合 | 原子時計, 宇宙技術, 医療用途 |
セシウムの産業的および科学的利用
しかし, セシウムは非常に柔らかい金属です; 反応性が高く、豊富に存在しないため、一般的な目的には使用されません。. 技術分野では非常に重要です.
1. 原子時計
セシウムは、人類が知る中で最も正確な時間を計測する装置の基礎的な構成要素です。, 原子時計. これらの時計は、セシウム原子の周波数が規則的に振動することで機能します。, このレベルの精度に匹敵するものはありません. GPSや通信には非常に必要です.
2. 石油とガスの探査
この流体は高密度で低粘度であるため、ボーリング孔へのサポートが向上します。. 同時に通常の流体に比べて環境への影響を低減します。.
3. 医療用途
セシウム同位体、特にセシウム 137 は、放射線療法の助けを借りてがん治療に応用されています。. それらは癌性組織の選択的排除を助け、周囲の組織への害を最小限に抑えます。.
4. 宇宙技術
金属イオンのセシウムは、宇宙船を推進するためのイオンスラスターに使用されます。. 反応しやすくイオン化する性質があるため、, セシウムは宇宙旅行で推力を生み出すのに最適な燃料です.
5. 光学ガラス
非放射性元素のセシウムは、特定の種類のガラスの品質を調整するために使用されます。. より鮮明で弾力性のあるものになります. セシウムベースのガラスは、反射面光学系の改善を必要とする強化レンズや機械に採用されています。.
6. 研究開発
セシウムは化学実験室で使用されています, 柔らかく反応性の高い金属であるため、物理実験や物理実験に使用できます。. このため, イオン化や金属結合の品質検査に役立ちます.
地球上で最も柔らかい金属であるセシウムの使用の長所と短所
それにもかかわらず, 見てわかるように, セシウムは他のアルカリ半金属よりも速く、はるかに優れた特性を持っています; しかし, この要素には、そのような材料の適用拡大を妨げる欠点があります.
1. 反応性
セシウムは反応性が非常に高く危険です. 不燃性です, 水と激しく反応する, 不活性雰囲気下で保存する必要があります. これらには、鉱物油または密封されたガラス製アンプルが含まれる場合があります。.
2. 希少性とコスト
セシウムは銀黄色のアルカリ金属で、比較的まれに使用され、主にポルサイトから得られます。. 珍しいですね, したがって, 費用がかかる, したがって、特定の分野でのみ有用です.
3. 毒性
セシウムの急性毒性は経口または吸入曝露を引き起こす可能性があります. 取り扱い中, 適切な措置を講じなければならない. それで, 健康への悪影響がないことを保証できます.
4. 環境への懸念
セシウムの採掘とセシウム 137 を含む放射性同位体の除去は、環境に配慮した作業とみなされます。. こういった理由から, 申請には非常に厳しい規則と管理がある.
金属の柔らかさの重要性
金属の柔らかさが特長です. 金属が従来何に使用されてきたのかを混乱させると考えられる可能性は非常に高いです。. 操作は比較的簡単です, または他の材料と形成および融合する, したがって、高い成形性が必要な場合に適しています。.
1. ソフトメタルの利点
- 他のデータベースやデータストレージ機器よりも処理と操作が簡単です.
- 複雑なパターンや差動切断装置に適しています.
- 通常合金に添加される, より強い金属に特定の特性を与える.
2. トレードオフ
柔らかい金属は壊れやすいため、慎重に扱う必要があります. より優れた機能を実現するために、他のより硬い素材と併用されることがよくあります。.
セシウムに関する興味深い事実
以下は、地球上で最も柔らかい金属であるセシウムに関するあまり知られていない事実です。:
- 発見: セシウムは、で発見された化学元素です。 1860 二人のドイツ人科学者による, ロバート・ブンス,火炎分光法によるnとグスタフ・キルヒホッフ.
- 名前の由来: このディテールは、セシウムが発光スペクトルに青い線を与えるため、空色を意味するラテン語にちなんで名付けられました。.
- 手に溶ける: 暖かい日にセシウムを扱うと面白いかもしれません。, あなたの手の中でとろけてしまいます – 化学元素の生命力を考えると、決して行うべきではない偉業です.
日常生活におけるその他の軟金属
セシウムは最も柔らかい金属ですが、, 柔らかさとさまざまな用途に適した他の金属もいくつかあります:
- 金: 比較的柔らかく、形を整えたり成形したりしやすい, 特にジュエリーやエレクトロニクスを製造する場所では.
- 鉛: 柔らかい, 密度が低い, バッテリーや放射線遮蔽に利用されています.
- 錫: 軽くて強靱な材質で耐食性に優れています。. さらに, コーティングや合金に一般的な用途があります.
結論
結論 – セシウムは世界的に最も柔らかい金属であり、その特徴により特定の用途では興味深い元素となります。. 高い化学反応性, 低密度, 展性, 希少性が高いため、原子時計などの用途に適しています。, 宇宙探査, そして薬. それにもかかわらず, その欠点, つまり. 大きな欠点と環境上の制約により使用が制限される. このほかにも, 通常の金属ではなく、特殊材料としていくつかの用途があります。.
セシウムやその他の軟金属は、科学を支える役割を高く評価できるようになる, とテクノロジー. それらは産業の成長において顕著な重要性を持っており、軟質材料であっても世界的な影響力を持ち得ることを示しています。. ソフトメタルと比較して, 非常に高い温度で, 摩耗または腐食環境, ジルコニアセラミックまたは アルミナセラミック 硬度の場合は代替として使用できます, 耐摩耗性と耐食性が重要な要件です. お問い合わせ 詳細については.
よくある質問
1. セシウムは常温で溶けますか??
もちろん, セシウムは室温よりわずかに高い温度で溶ける可能性があります, 28.5 ℃ (83.3 °F). 暖かい気候では液体になる可能性があります.
2. 元素について何を知っていますか, セシウム?
セシウムは石油および天然ガス産業の掘削液として原子時計の生成に役立ちます. それで, 宇宙ブースターロケットに動力を供給できる, がん治療, および精密光学ガラスの製造.
3. セシウムは安全に扱えますか?
セシウムは反応性が高いので、, さまざまな用途には不活性環境でのみ使用してください。. 水に非常に敏感で、水に触れると激しく反応します。.
4. セシウムは金や鉛などの他の軟金属と比べてどのような位置にあるのでしょうか?
柔らかい元素としての金や鉛と比較して, セシウムは原子配置の関係で比較的柔らかい. それにもかかわらず, 金と鉛はより安定した金属であり、日常生活に幅広い用途があります。.