Materiais de cerâmica são essenciais em indústrias que variam de aeroespacial a eletrônica, Mas muitas pessoas costumam perguntar: “A cerâmica tem um ponto de fusão específico?” A resposta curta é não - não um único ponto de fusão, mas um grande alcance, dependendo da composição do material.
Este guia abrangente explora tudo o que você precisa saber sobre pontos de fusão de cerâmica, seus limites de temperatura, comportamento de choque térmico, e aplicações práticas.
O que é material de cerâmica?
Materiais cerâmicos são uma classe ampla de inorgânicos, sólidos não metálicos. Ao contrário de metais e polímeros, cerâmica:
Carecem de ligações de carbono-hidrogênio, o que significa que eles estão livres de substâncias orgânicas.
Não contém elementos metálicos, embora possam estar ligados a metais em forma de óxido.
Consistem principalmente em compostos como óxidos, nitreto, carbonetos, silicatos, e outros minerais.
Propriedades -chave da cerâmica
A cerâmica exibe uma variedade de propriedades únicas e valiosas:
Alta dureza e força
Resistência ao desgaste e abrasão
Excelente isolamento elétrico
Excelente resistência térmica
Estabilidade química em ambientes agressivos
Os materiais de cerâmica são comumente divididos em duas categorias:
Cerâmica tradicional: Cerâmica, porcelana, tijolos
Cerâmica avançada: Usado no aeroespacial, dispositivos médicos, eletrônicos, e ferramentas de corte
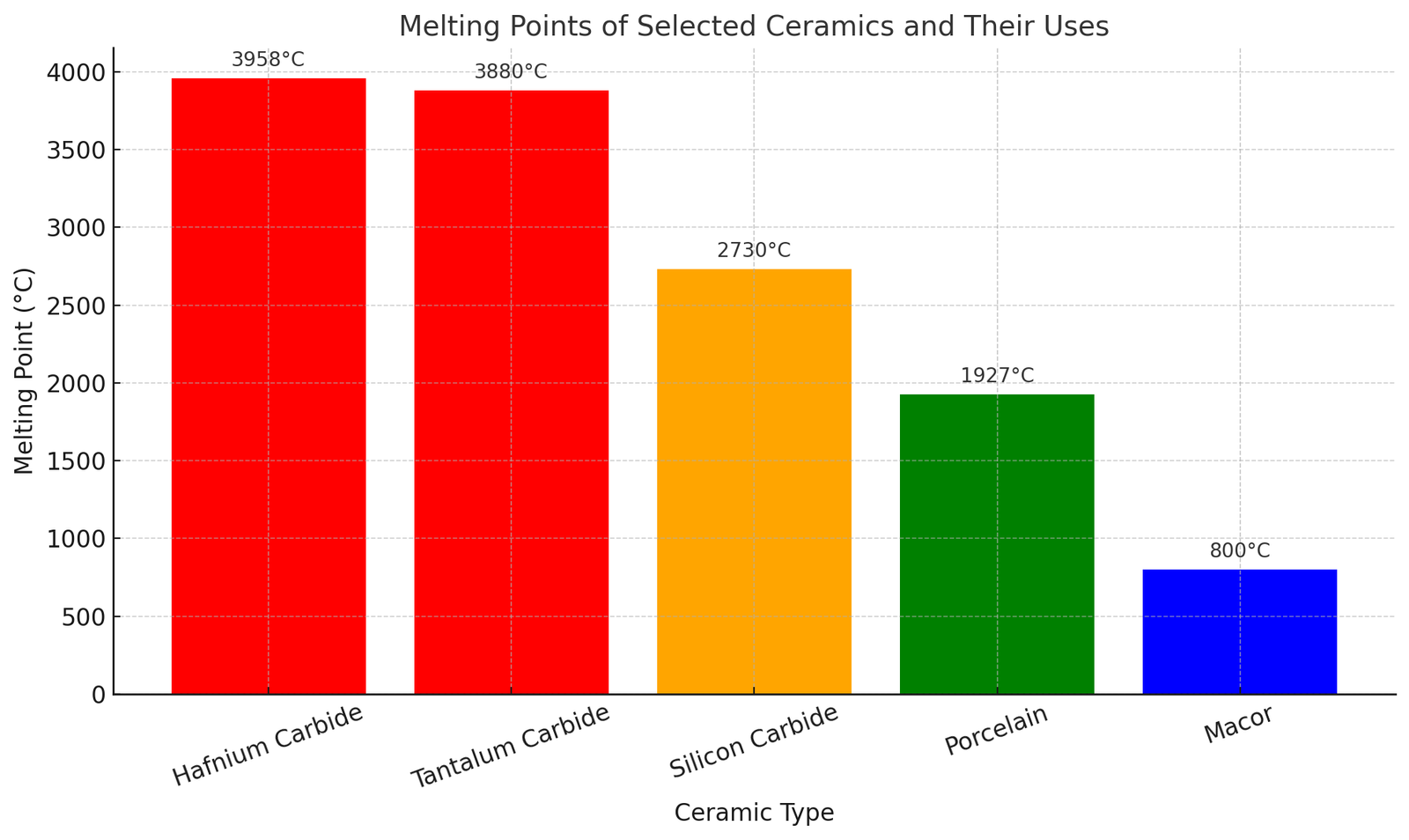
Exemplos de materiais de cerâmica e seus pontos de fusão
O ponto de fusão dos materiais de cerâmica depende significativamente de sua composição química. Aqui está uma tabela listando várias cerâmicas com seus pontos de fusão aproximados:
| Material de cerâmica | Ponto de fusão (°C) | Observações |
| Carboneto de hafnium | ≈ 3,958 | Um dos pontos de fusão mais altos de qualquer composto conhecido |
| Carboneto de Tantalum | ≈ 3,880 | Excelente para aplicações aeroespaciais e de resistência a calor |
| Carboneto de titânio | ≈ 3,160 | Extrema dureza e força; usado em ferramentas de corte de alto desempenho |
| Carboneto de tungstênio | ≈ 2,870 | Dureza excepcional e resistência ao desgaste |
| Carboneto de silício | ≈ 2,730 | Resistência ao choque térmico; Ideal para fornos de alta temperatura e semicondutores |
| Zircônia (Zro₂) | ≈ 2,700 | Alta resistência à fratura; Usado em revestimentos dentários e térmicos |
| Nitreto de alumínio | ≈ 2,200 | Boa condutividade térmica e isolamento elétrico |
| Alumina (Al₂o₃) | ≈ 2,045 | Comum em substratos eletrônicos e velas de ignição |
| Mullite | ≈ 1,840 | Frequentemente usado em revestimentos refratários |
| Silicato de alumina | ≈ 1,790 | Popular em componentes de isolamento e forno |
| Porcelana | ≈ 1,927 | Cerâmica tradicional para sofrimento sanitário, azulejos, e isoladores elétricos |
| Sílica (Quartzo) | ≈ 1,710 | Excelente estabilidade térmica e resistência à devitrificação |
| China de ossos | ≈ 1,670 | Uma forma refinada de porcelana com alta resistência e translucidez |
| Tijolos de fogo | ≈ 1.540-2.200 | Projetado para desempenho refratário em fornos e fornos |
| Cerâmica de vidro | ≈ 850-1.723 | Conhecido por alta resistência e estabilidade dimensional |
| Macor | ≈ 800 | Cerâmica de vidro máquinável usada em aplicações de precisão |
🔎 Observação: Esses valores são aproximados. Os pontos de fusão exatos podem variar dependendo de aditivos e técnicas de processamento.
🔥 Como o ponto de fusão de uma cerâmica decide para que é usado
🛠️ Alto ponto de fusão = empregos pesados
Algumas cerâmicas podem lidar com calor extremo - até 3.000 ° C ou mais! Esses materiais super-duplos são perfeitos para indústrias que lidam com temperaturas muito altas, como:
Aeroespacial - Peças do motor de foguete, escudos de calor para veículos de reentrada
Fundições e plantas de aço - revestimentos e moldes do forno
Reatores nucleares -Componentes resistentes ao calor para processamento de combustível
Esses tipos de cerâmica incluem Carboneto de Tantalum e carboneto de hafnium, frequentemente chamado cerâmica de temperatura ultra-alta.
🍽️ Ponto de fusão média = coisas do dia a dia
Cerâmica com pontos de fusão moderados, Diga cerca de 1.500-2.000 ° C., são usados em itens mais familiares:
Painéis e pratos - como porcelana e osso na China
Ladrilhos e acessórios de banheiro - durável e fácil de limpar
Isoladores - Usado em aquecedores e fios elétricos
Eles são fortes, tolerante ao calor, e não se decompõe facilmente-mas eles não são feitos para calor super -treme.
🧪 Ponto de fusão baixo = usos especiais
Cerâmica que derrete a temperaturas mais baixas (Cerca de 800-1.200 ° C.), Como alguns cerâmica de vidro e Macor, são frequentemente usados para tarefas mais delicadas:
Ferramentas de laboratório - onde a precisão é importante
Peças máquinas - onde a modelagem exata é importante
Implantes médicos - onde superfícies suaves e segurança são fundamentais
Mesmo que eles não possam levar tanto calor, essas cerâmicas são escolhidas para seus outros benefícios - como ser fácil de moldar, forte sob pressão, ou seguro dentro do corpo.
Resumindo?
Se uma cerâmica derrete a uma temperatura realmente alta, provavelmente é usado em alto calor, empregos industriais. A cerâmica de ponto de fusão mais baixa ainda é super útil - mas em lugares onde as coisas não ficam bastante tão quente.
Entendendo o limite de temperatura cerâmica
Materiais de cerâmica não compartilham um único, ponto de fusão unificado. Em vez de, Seu desempenho térmico é determinado por:
Estrutura cristalina
Força de ligação
Composição elementar
Aditivos e impurezas
Por exemplo:
Carboneto de titânio suporta até 3.160 ° C.
Tijolos de fogo, por contraste, operar dentro de 1.540-2.200 ° C..
Essa variabilidade é por que os engenheiros devem selecionar cerâmica com base no Requisitos térmicos específicos de aplicativos.
Choque térmico em cerâmica
Enquanto a maioria da cerâmica tolera altas temperaturas, sua resposta a choque térmico é outra história.
O que é choque térmico?
O choque térmico ocorre quando um material passa por um mudança rápida de temperatura, criando estresse interno devido à expansão ou contração desigual. Para cerâmica, que são tipicamente quebradiços, Isso pode resultar em:
Rachadura
Espalhamento
Fratura completa
Resistência ao choque térmico
Algumas cerâmicas gostam carboneto de silício e zircônia têm resistência de choque térmico relativamente alta devido à sua condutividade e resistência térmica. Outros, como cerâmica de vidro, são projetados para suportar variações repentinas de temperatura.
Fluência em cerâmica
Apesar de suas altas temperaturas de fusão, cerâmica ainda pode sofrer deformação de fluência- especialmente abaixo estresse mecânico sustentado a temperaturas elevadas. Este fenômeno é fundamental em aplicações aeroespaciais e nucleares.
Perguntas frequentes
1. Que temperatura pode suportar cerâmica?
Dependendo do material:
Carboneto de silício: ≈ 2.730 ° C.
Alumina: ≈ 2.045 ° C.
Tijolos de fogo: até ~ 2.200 ° C
2. A que temperatura vai rachadura de cerâmica?
A rachadura de cerâmica depende mais de choque térmico e estresse mecânico do que apenas temperatura. Aquecimento ou resfriamento rápido é a causa típica.
3. Por que a cerâmica tem um ponto de fusão alto?
Cerâmica é composta de fortes ligações iônicas e covalentes, que requerem energia significativa para quebrar. Os átomos de luz e alta energia de ligação tornam a cerâmica extremamente resistente ao calor.
4. Pode quebrar cerâmica devido ao calor?
Sim. Aquecimento rápido ou resfriamento pode causar estresse interno, levando à fratura. Mesmo que a cerâmica não atinja seu ponto de fusão, O choque térmico ainda pode causar danos.
5. Quais materiais de cerâmica têm os pontos de fusão mais altos?
Carboneto de hafnium: ≈ 3.958 ° C.
Carboneto de Tantalum: ≈ 3.880 ° C.
Estes são classificados como cerâmica de temperatura ultra-alta, frequentemente usado em bicos de foguetes e componentes de voo hipersônicos.
6. Pode quebrar cerâmica em um freezer?
Sim. Assim como com altas temperaturas, uma queda repentina de temperatura pode causar rachaduras devido a Contração térmica- especialmente se a cerâmica não for projetada para esse estresse.
Conclusão
Materiais de cerâmica são uma classe diversificada de compostos com Pontos de fusão excepcionalmente altos. No entanto, Esses pontos de fusão variam muito - de 800° C a quase 4.000 ° C- dependendo da composição e estrutura química da cerâmica.
Entendendo o Propriedades térmicas, ponto de fusão, e resistência ao choque térmico de cerâmica é fundamental para selecionar o material certo para industrial, médico, aeroespacial, ou aplicações de consumidores.
Escolhendo a cerâmica certa para seus requisitos térmicos e mecânicos, Você pode obter um excelente desempenho até mesmo nos ambientes mais severos.