Ceramic materials are essential in industries ranging from aerospace to electronics, but many people often ask: “Do ceramics have a specific melting point?” The short answer is no—not a single melting point, but rather a wide range depending on the material’s composition.
This comprehensive guide explores everything you need to know about ceramic melting points, their temperature limits, thermal shock behavior, and practical applications.
What is Ceramic Material?
Ceramic materials are a broad class of inorganic, non-metallic solids. Unlike metals and polymers, ceramics:
Lack carbon-hydrogen bonds, meaning they are free of organic substances.
Contain no metallic elements, though they may be bonded with metals in oxide form.
Consist mainly of compounds like oxides, nitrides, carbides, silicates, and other minerals.
Key Properties of Ceramics
Ceramics exhibit a range of unique and valuable properties:
High hardness and strength
Wear and abrasion resistance
Excellent electrical insulation
Outstanding thermal resistance
Chemical stability in harsh environments
Ceramic materials are commonly divided into two categories:
Traditional ceramics: Pottery, porcelain, bricks
Advanced ceramics: Used in aerospace, medical devices, electronics, and cutting tools
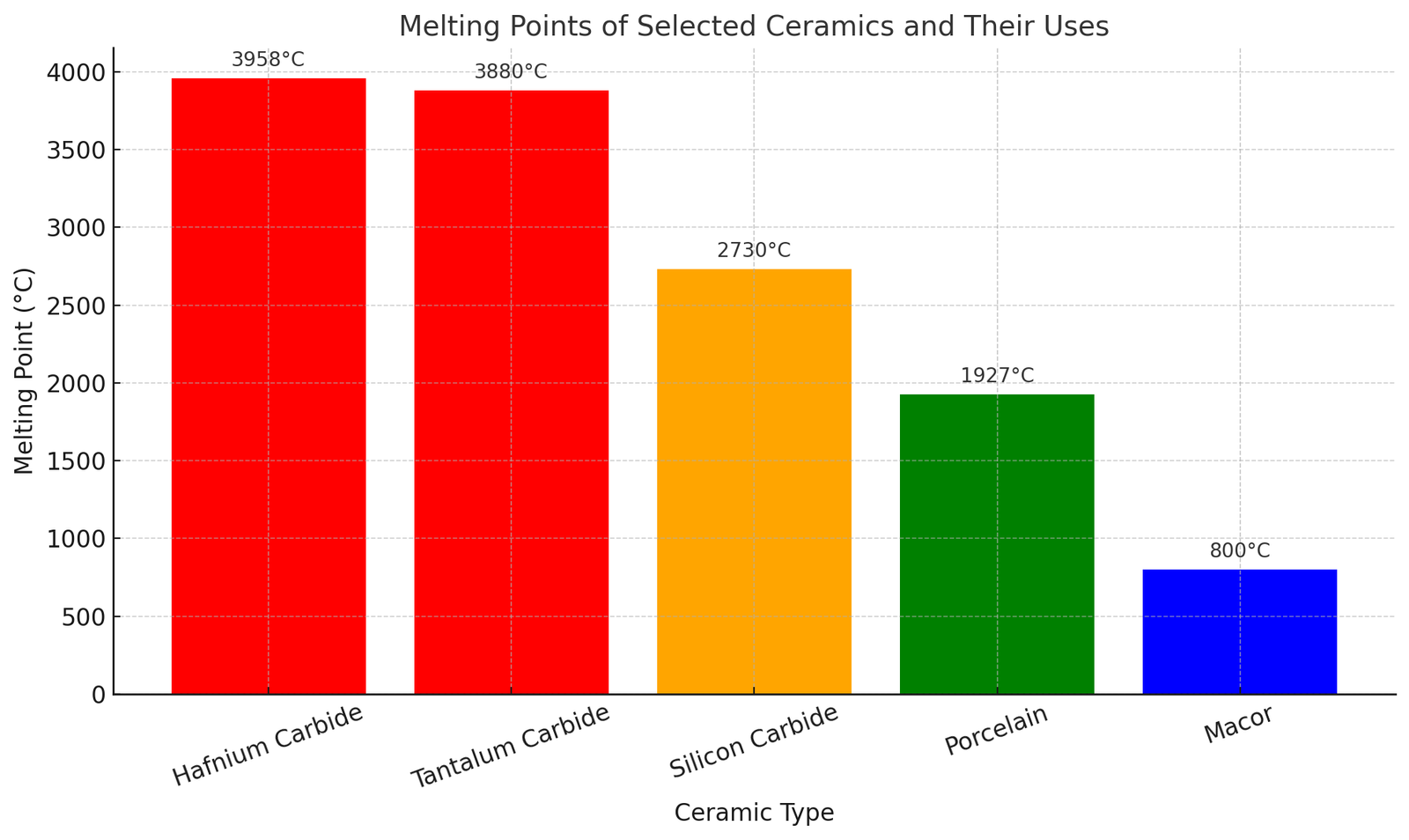
Examples of Ceramic Materials and Their Melting Points
The melting point of ceramic materials depends significantly on their chemical makeup. Here’s a table listing various ceramics with their approximate melting points:
| Ceramic Material | Melting Point (°C) | Remarks |
| Hafnium carbide | ≈ 3,958 | One of the highest melting points of any known compound |
| Tantalum carbide | ≈ 3,880 | Excellent for aerospace and high-heat resistance applications |
| Titanium carbide | ≈ 3,160 | Extreme hardness and strength; used in high-performance cutting tools |
| Tungsten carbide | ≈ 2,870 | Exceptional hardness and wear resistance |
| Silicon carbide | ≈ 2,730 | Thermal shock resistance; ideal for high-temperature furnaces and semiconductors |
| Zirconia (ZrO₂) | ≈ 2,700 | High fracture toughness; used in dental and thermal barrier coatings |
| Aluminum nitride | ≈ 2,200 | Good thermal conductivity and electrical insulation |
| Alumina (Al₂O₃) | ≈ 2,045 | Common in electronic substrates and spark plugs |
| Mullite | ≈ 1,840 | Often used in refractory linings |
| Alumina silicate | ≈ 1,790 | Popular in insulation and kiln components |
| Porcelain | ≈ 1,927 | Traditional ceramic for sanitaryware, tiles, and electrical insulators |
| Silica (Quartz) | ≈ 1,710 | Excellent thermal stability and resistance to devitrification |
| Bone China | ≈ 1,670 | A refined form of porcelain with high strength and translucency |
| Fire bricks | ≈ 1,540–2,200 | Engineered for refractory performance in furnaces and kilns |
| Glass ceramics | ≈ 850–1,723 | Known for high toughness and dimensional stability |
| Macor | ≈ 800 | Machinable glass-ceramic used in precision applications |
🔎 Note: These values are approximate. Exact melting points can vary depending on additives and processing techniques.
🔥 How the Melting Point of a Ceramic Decides What It’s Used For
🛠️ High Melting Point = Heavy-Duty Jobs
Some ceramics can handle extreme heat—up to 3,000°C or more! These super-tough materials are perfect for industries that deal with very high temperatures, like:
Aerospace – Rocket engine parts, heat shields for reentry vehicles
Foundries and steel plants – Furnace linings and molds
Nuclear reactors – Heat-resistant components for fuel processing
These types of ceramics include tantalum carbide and hafnium carbide, often called ultra-high temperature ceramics.
🍽️ Medium Melting Point = Everyday Stuff
Ceramics with moderate melting points, say around 1,500–2,000°C, are used in more familiar items:
Cookware and dishes – Like porcelain and bone china
Tiles and bathroom fixtures – Durable and easy to clean
Insulators – Used in electric heaters and wires
They’re strong, heat-tolerant, and don’t break down easily—but they’re not meant for super-extreme heat.
🧪 Low Melting Point = Special Uses
Ceramics that melt at lower temperatures (around 800–1,200°C), like some glass ceramics and Macor, are often used for more delicate tasks:
Laboratory tools – Where precision matters
Machinable parts – Where exact shaping is important
Medical implants – Where smooth surfaces and safety are key
Even though they can’t take as much heat, these ceramics are chosen for their other benefits—like being easy to shape, strong under pressure, or safe inside the body.
Bottom Line?
If a ceramic melts at a really high temperature, it’s likely used in high-heat, industrial jobs. Lower melting point ceramics are still super useful—but in places where things don’t get quite as hot.
Understanding Ceramic Temperature Limit
Ceramic materials do not share a single, unified melting point. Instead, their thermal performance is determined by:
Crystal structure
Bonding strength
Elemental composition
Additives and impurities
For example:
Titanium carbide withstands up to 3,160°C.
Fire bricks, by contrast, operate within 1,540–2,200°C.
This variability is why engineers must select ceramics based on the application-specific thermal requirements.
Thermal Shock on Ceramic
While most ceramics tolerate high temperatures, their response to thermal shock is another story.
What is Thermal Shock?
Thermal shock occurs when a material undergoes a rapid temperature change, creating internal stress due to uneven expansion or contraction. For ceramics, which are typically brittle, this can result in:
Cracking
Spalling
Complete fracture
Thermal Shock Resistance
Some ceramics like silicon carbide and zirconia have relatively high thermal shock resistance due to their thermal conductivity and toughness. Others, such as glass ceramics, are engineered to endure sudden temperature variations.
Creep in Ceramics
Despite their high melting temperatures, ceramics may still undergo creep deformation—especially under sustained mechanical stress at elevated temperatures. This phenomenon is critical in aerospace and nuclear applications.
FAQs
1. What Temperature Can Ceramic Withstand?
Depending on the material:
Silicon carbide: ≈ 2,730°C
Alumina: ≈ 2,045°C
Fire bricks: up to ≈ 2,200°C
2. At What Temperature Will Ceramic Crack?
Ceramic cracking depends more on thermal shock and mechanical stress than just temperature. Rapid heating or cooling is the typical cause.
3. Why Does Ceramic Have a High Melting Point?
Ceramics are composed of strong ionic and covalent bonds, which require significant energy to break. The light atoms and high bonding energy make ceramics extremely heat-resistant.
4. Can Ceramic Break Due to Heat?
Yes. Rapid heating or cooling can cause internal stress, leading to fracture. Even if the ceramic doesn’t reach its melting point, thermal shock can still cause damage.
5. Which Ceramic Materials Have the Highest Melting Points?
Hafnium carbide: ≈ 3,958°C
Tantalum carbide: ≈ 3,880°C
These are classified as ultra-high temperature ceramics, often used in rocket nozzles and hypersonic flight components.
6. Can Ceramic Break in a Freezer?
Yes. Just as with high temperatures, a sudden drop in temperature can cause cracking due to thermal contraction—especially if the ceramic is not engineered for such stress.
Conclusion
Ceramic materials are a diverse class of compounds with exceptionally high melting points. However, these melting points vary widely—from 800°C to nearly 4,000°C—depending on the ceramic’s chemical composition and structure.
Understanding the thermal properties, melting point, and thermal shock resistance of ceramics is critical for selecting the right material for industrial, medical, aerospace, or consumer applications.
By choosing the right ceramic for your thermal and mechanical requirements, you can achieve outstanding performance in even the harshest environments.