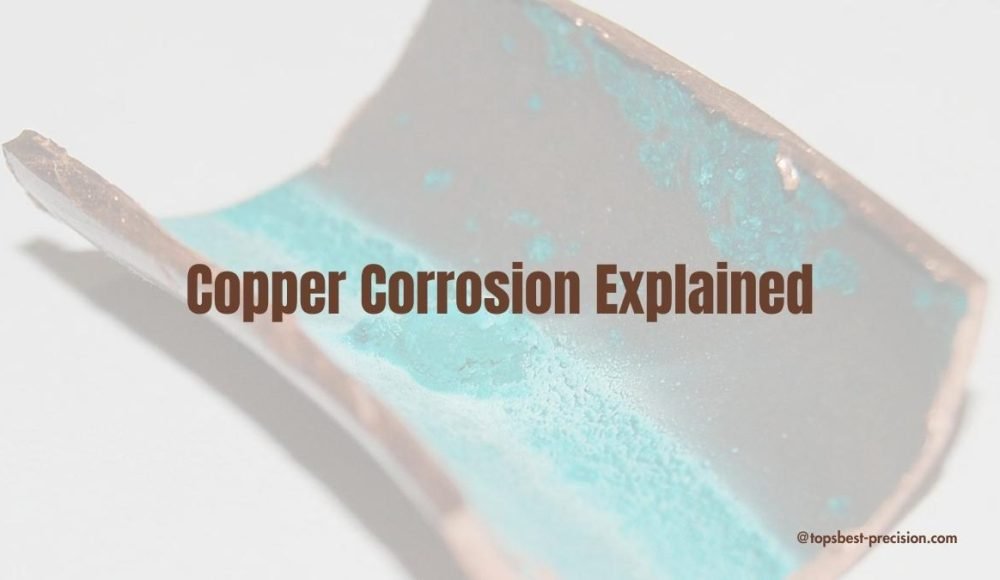
Copper corrosion means copper deterioration due to moisture, and air exposure. Typically, copper corrodes, and turns into tarnishes black( a green film). Keep on reading to learn more about copper corrosion.
Does Copper Rust?
Copper does not rust like iron does. The term rust only emphasizes for iron corrosion products. Copper, on the other hand, corrodes and forms a green layer(Patina). Generally, patina is copper carbonate CuCO₃. It forms when Cu is reacted with air, and water after long exposure. The patina layer may look unattractive, but it serves a protective purpose. It prevents further corrosion of base(underlying) metal. As a result, copper can serve a long time with minimal maintenance.
How Copper Patina Formation Occurs?
Copper reacts with atmospheric oxygen to form cuprous oxide. Copper rust is a reddish-brown layer of Cu2O. This layer evolves by the process of electrochemical interaction with air. While iron turns to rust, copper does not corrode, or as simply said, it does not rust, it oxidizes. Oxygen acts on copper to prevent the formation of a compound known as copper oxide. This oxide has a property different from that of iron oxide Fe2O3. Copper oxide, conversely, does not corrode as fast as rust does. It acts as a barrier that gradually becomes thicker as it accumulates. Finally, this layer changes to copper carbonate in this process. The top layer is known as the patina.
Factors Contribute To Copper Corrosion
Copper corrosion can be affected by several factors. It’s quite imperative to know those factors to avoid and minimize them.
Environmental Exposure
Corrosion of copper is hastened in areas of salinity, heat, and contentious chemicals such as acids. These conditions cause a chemical action with the copper, resulting in the surface being weakened and exposed to more harm than before.
Electrical Current Effects
Although both direct and AC electrical currents can raise the corrosion rate of copper pipes. These pipes are frequently buried underground. The existence of electrical potential differences aggravates the localized corrosion phenomena.
Galvanic Corrosion
Galvanic corrosion is where the copper reacts with dissimilar materials. For instance, steel. Such an interaction gives the formation of an electrochemical cell. This accommodates a faster rate of copper corrosion because the conductivity is equally not the same.
Aggressive Soil Conditions
Chlorides, sulfates, and ammonia compounds are commonly present in soils. These natural ingredients can pose a great danger to copper corrosion. Mobilizing these aggressive soil conditions, coupled with moisture retention, invariably results in severe corrosion loss over some time.
Acidic Contact
The presence of organic and inorganic acids poses a major threat to the copper quality. These acids get through the oxide layer and go on destroying the material at a faster rate. Q
Corrosion Fatigue
Fatigue is caused by the fluctuations of stress that occur in copper materials. The rapid flow of fluids and fluctuation in temperatures fosters localized erosion that tends to result in early failure of the material.
Oxygen Exposure
The oxidation rates of copper surfaces are likely to be increased with a rise in oxygen levels in the atmosphere. This accelerates the formation of corrosive compounds by simultaneously degrading the already poor mechanical integrity of the metal.
Types of Copper Corrosion
The copper can noticeably corrotype in certain ways. Both types are affected by environmental and chemical conditions.
Uniform Corrosion
Uniform corrosion occurs gradually over the surface and impacts the whole surface area equally at one point in time. This type most often leads to a gradual reduction in the thickness of the metal. It’s especially common where copper is exposed to moisture. Acidic conditions also increase the rate of this uniform degradation process with the fibers.
Pitting Corrosion
Pitting corrosion causes the formation of pits on the copper surface. Chiefly, it’s provoked by such ions as chlorides with an aggressive effect. These pits can become severely deep to a point where the structural soundness of the pits can happen. Pitting corrosion is usually worsened by the presence of stagnant water.
Crevice Corrosion
Crevice corrosion happens in narrow spaces and narrow gaps. The condition allows static solutions to form, and expressly delimit oxygen access. In such circumstances, corrosive agents accumulate in the system. As a result, a concentration attack takes place leading to localized corrosion. It most commonly occurs around fasteners, gaskets, and pipe joints.
Galvanic Corrosion
Galvanic corrosion is a phase by which copper encounters other types of metals. The contact develops a galvanic cell in the presence of an electrolyte(a conductive solution). The one among the two metals with higher reactivity corrodes at a faster pace. However, you must note that copper can corrode locally, which will decrease the useful material length.
Dezincification
Dezincification occurs only in brass. Brass is a mixture (alloy) of copper and zinc. During dezincification, one of the couples or the components, the zinc elements is always seen to disintegrate. This results in a porous structure of copper which is comparatively weaker than the initial structure. Dezincification is particularly undesirable in applications where a high degree of mechanical performance is required.
Intergranular Corrosion
Intergranular corrosion takes place at the grain boundaries in the copper. The type usually originates from inadequate heat treatment processes during production. It can also be encouraged by exposure to particular corrosive conditions as explained above. The weakening of the material can lead to catastrophic failures.
Stress Corrosion Cracking
Stress corrosion cracking is a stress product and corrosive agent at the surface of the material. It forms some small openings in the copper material, commonly referred to as ruptures. Usually, ammonia contributes to this problem. Moreover, high temperatures can also lead to fracturing issues.
Different forms of copper corrosion are characterized by different difficulties and processes. These are aspects that we have to consider – and understand – so we know how to proceed in the matter of prevention. Corrosion is mainly influenced by the physical, and chemical features of copper alloys, environmental conditions, and stresses exerted.
Examples of Corrosion-Resistant Alloys
Aluminum Brass: These alloys are very immune to impingement corrosion, especially in high-velocity saltwater applications.
Aluminum Bronze: It has been used widely for its ability to resist chemical attacks, particularly from sulfite solutions.
Copper-Silicon Alloys: These alloys offer high resistance to stress-corrosion cracking in comparison with conventional brass.
Nickel Silvers: They tend to provide excellent resistance against corrosion from both fresh and marine water.
Conclusion
In summary, it’s imperative to preserve copper and understand how it corrodes. Corrosion appears in many forms, ranging from crevice formation to patina layer. Both uniform, pitting, and galvanic corrosion represent specific types of corrosion and demand specific measures for their mitigation. By this, we can identify what leads to corrosion and then work out how to avoid it. In general, the information regarding the mechanism of corrosion of copper not only helps to preserve magnificent architecture and relics. Also, it helps increase the efficiency of using copper in different fields.
FAQs
Q1. What is copper corrosion, and its causes?
Copper corrosion refers to the loss of material properties. Copper is attributable to the chemical action of its environment whereby it distributes its components under the impact of dampness and air. The process forms a greenish patina on the metal surface.
Q2. How does the patina form on copper material?
Copper patina forms when copper is oxidized by oxygen, carbon dioxide, and moisture. The additional layer of patina helps stop more corrosion from taking place, a key part of copper’s endurance outdoors.
Q3. Can copper corrosion be prevented?
Yes, protective measures include the application of anti-corrosive paints, the use of corrosion retardants, and providing proper drainage to minimize conditions. Other preventive takeups include copper washing, and polishing to improve the look and longevity of the copper surface.
Q4.How does copper corrosion Influence the construction sector?
When copper corrodes it can weaken structures in areas such as plumbing and roofing. Therefore it is important to understand and control corrosion to extend the life and safety of part’s structures.